
结直肠癌(CRC)是全球发病率和死亡率均位居前列的恶性肿瘤之一,且其疾病负担正在持续增加。结直肠癌的发生和发展是一个多基因、多信号通路协同作用的复杂过程,驱动基因突变(如APC、KRAS等)引发关键通路失调,伴随表观遗传改变和肿瘤微环境重塑,最终促进正常结直肠细胞向恶性肿瘤转化。
1.1 Wnt/β-catenin信号通路
Wnt/β-catenin通路是结直肠癌的核心致癌通路,约80%的结直肠癌患者中此通路出现异常。正常情况下,β-catenin通过GSK-3β介导的磷酸化途径被降解,保持通路稳定。APC基因突变或Axin功能缺失会导致β-catenin在细胞质中蓄积,并进入细胞核与Tcf/Lef转录因子结合,激活MYC、Cyclin D1等增殖相关基因,成为结直肠癌早期的标志性事件。
1.2 EGFR信号通路
表皮生长因子受体(EGFR)是一个跨膜受体,通过与其配体结合激活下游的Ras-Raf-MEK-ERK及PI3K-Akt-mTOR通路,调控细胞增殖、迁移与存活。在结直肠癌中,约60%的病例中EGFR蛋白表达过高,且KRAS突变可导致该通路的配体非依赖性持续激活,这是研究EGFR调控网络的一个重要切入点。
1.3 PI3K/Akt/mTOR信号通路
PI3K/Akt/mTOR通路通过PIK3CA基因突变或PTEN基因缺失异常激活,参与肿瘤细胞的代谢重编程和耐药性形成。该通路中的关键分子,如Akt和mTOR的活性变化,与结直肠癌细胞对外部环境的适应能力密切相关,是研究肿瘤细胞生存机制及联合治疗策略的关键靶点。
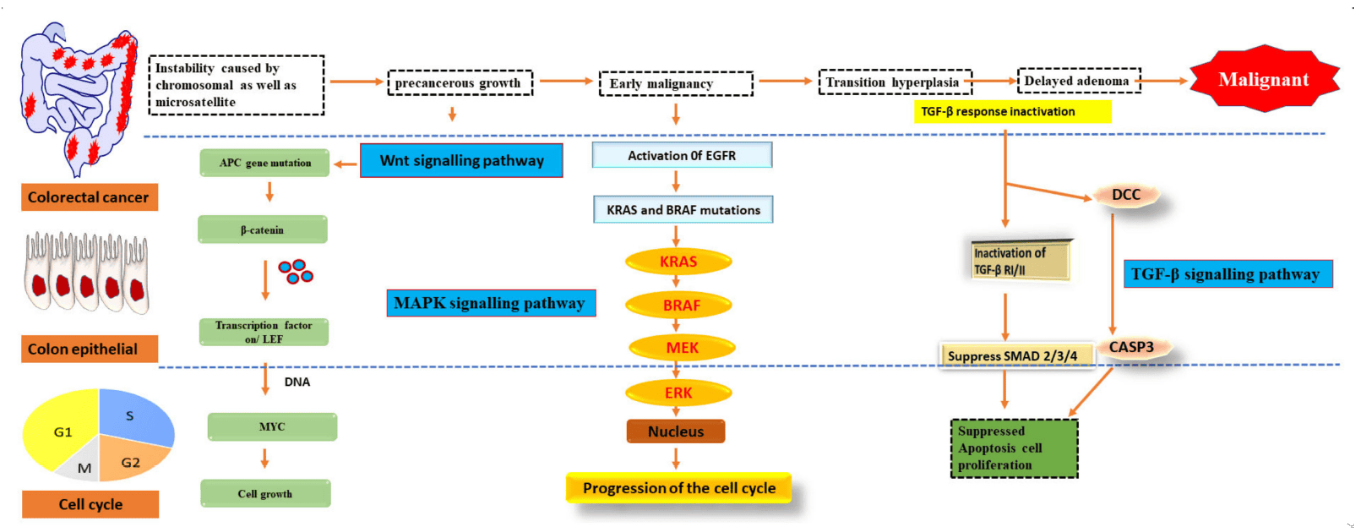
图1. Pathways of signalling that have been genetically changed by CRC.
2.1 靶向调控机制研究
当前科研聚焦于结直肠癌经典驱动基因的靶向调控,例如KRAS突变(尤其是G12C亚型)的小分子抑制剂的研发,探索其与KRAS蛋白的结合机制及如何阻断下游通路。此外,BRAF突变相关通路的协同调控研究也在深入进行,目标是明确多靶点联合干预的作用网络。
2.2 免疫相关机制探索
关于结直肠癌的免疫微环境的研究正在深入,重点关注PD-1/PD-L1分子的表达调控及MSI-H/dMMR表型与免疫反应的关系。研究还发现,POLE/POLD1突变影响肿瘤免疫原性,新型免疫调控分子的筛选为理解结直肠癌免疫逃逸机制提供了新的视角。
2.3 早期分子机制挖掘
早期筛查领域的研究主要集中在早期结直肠癌的分子标志物,例如分析粪便样本中的KRAS突变、BMP3/NDRG4甲基化等特征,探索它们与结直肠上皮细胞恶变的关系,并通过细胞模型和动物模型验证这些分子标志物在结直肠癌早期阶段的变化规律。
|
靶点 |
生物学功能 |
在结直肠癌中的病理作用 |
科研应用场景 |
|
APC |
作为Wnt/β-catenin通路负调控因子,通过结合 β-catenin促进其降解,维持通路稳态;参与细胞黏附与迁移调控 |
胚系/体细胞突变导致功能缺失,β-catenin无法降解而核内蓄积,激活MYC等增殖基因,推动结直肠上皮细胞恶性转化,是早期癌变核心驱动事件 |
1. 构建APC突变细胞/动物模型(如ApcMin/+ 小鼠),研究早期癌变机制; |
|
KRAS |
作为GTP酶,在EGFR等通路中扮演“分子开关”,结合GTP时激活下游MAPK/PI3K通路,调控细胞增殖与分化 |
热点突变(12/13/59/61/117/146 位)导致GTP酶活性丧失,通路持续激活,促进癌细胞增殖、迁移及耐药性形成;与不良预后相关 |
1. 构建不同KRAS突变亚型细胞模型,分析突变功能差异; |
|
EGFR |
跨膜酪氨酸激酶受体,结合EGF/TGF-α后二聚化,激活下游MAPK/PI3K通路,调控细胞生长、存活及血管生成 |
蛋白过表达或通路异常激活,增强癌细胞增殖与侵袭能力;KRAS突变可导致EGFR通路不依赖配体持续激活,参与治疗耐药 |
1. 采用Co-IP技术研究EGFR与下游分子的蛋白互作; |
|
BRAF V600E |
丝氨酸/苏氨酸激酶,作为MAPK通路关键分子,磷酸化MEK以激活通路,调控细胞增殖与凋亡平衡 |
热点突变导致激酶活性显著增强,持续激活MAPK通路,加速结直肠癌进展;与肿瘤高侵袭性、预后差相关 |
1. 构建BRAF V600E突变移植瘤模型,研究通路激活对肿瘤生长的影响; |
|
VEGF |
血管内皮细胞特异性生长因子,结合VEGFR后促进内皮细胞增殖、迁移及血管形成,维持组织血液供应 |
肿瘤微环境中过量表达,诱导新生血管生成,为癌细胞提供营养与氧气,促进肿瘤生长及远处转移;与肿瘤分期进展相关 |
1. 采用血管内皮细胞模型,研究VEGF对血管生成的调控效应; |
|
PD-L1 |
免疫检查点分子,表达于免疫细胞/肿瘤细胞表面,结合PD-1后抑制T细胞活化,维持免疫耐受 |
肿瘤细胞高表达PD-L1,与T细胞PD-1结合抑制抗肿瘤免疫应答,导致免疫逃逸;与MSI-H表型存在一定关联 |
1. 采用免疫细胞共培养模型,研究PD-L1对T细胞功能的影响; |
|
HER2 |
跨膜酪氨酸激酶受体,与其他HER家族成员二聚化后激活PI3K/Akt通路,调控细胞增殖与存活 |
基因扩增或蛋白过表达,导致通路异常激活,促进癌细胞增殖、迁移及耐药性形成;在HER2阳性亚型中为核心驱动事件 |
1. 建立HER2过表达细胞模型,研究其对癌细胞恶性表型的影响; |
|
Claudin18.2 |
紧密连接蛋白家族成员,参与维持上皮细胞间紧密连接完整性,调控细胞极性与物质转运 |
表达异常导致细胞间连接破坏,增强癌细胞侵袭能力;在部分结直肠癌中高表达,与肿瘤转移潜能相关 |
1. 采用Transwell模型,研究Claudin18.2对癌细胞侵袭能力的影响; |
|
类别 |
产品名称 |
货号 |
应用 |
|
蛋白 |
Recombinant Human APC Protein, N-His |
HB932012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
Recombinant Human CTNNB1 Protein, N-His |
HW518012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human GSK3B Protein, N-His |
HW521012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human GSK3B Protein, N-GST |
HW521022 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human TNKS2 Protein, N-His |
HV525012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human KRAS/K-Ras 2 (G12C) Protein, N-GST |
HF904042 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human KRAS Protein, N-His |
HF904012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human EGFR/ERBB1/HER1 Protein, N-His |
HF004032 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Rat EGFR/ERBB1/HER1 Protein, N-His |
RF004012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human EGFR Protein, N-His |
HF004012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human BRAF Protein, N-His |
HB617012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human VEGFD Protein, N-His |
HT285012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human VEGFC Protein, N-His |
HW305012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human CD340/ERBB2/HER2/NEU Protein, C-Fc |
HY286021 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
Recombinant Human CLDN18/Claudin-18 Protein, N-His |
HX126012 |
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress |
|
|
抗体 |
Anti-GSK3B Polyclonal Antibody |
HW521014 |
ELISA, IHC, WB |
|
Anti-CTNNB1 Polyclonal Antibody |
HW518014 |
ELISA, IHC, WB |
|
|
Anti-Human CTNNB1 Nanobody |
HW518013 |
ELISA, IF, IHC, IP, SPR, WB |
|
|
Anti-TNKS2 Polyclonal Antibody |
HV525014 |
ELISA, IHC, WB |
|
|
Anti-KRAS Polyclonal Antibody |
HF904014 |
ELISA, IHC, WB |
|
|
Anti-Human KRAS/K-Ras 2 Antibody (SAA1513) |
HF904013 |
ELISA |
|
|
Anti-Human EGFR/ERBB1/HER1 Antibody (11F8) |
HF004107 |
ELISA, FCM, WB |
|
|
Resaerch Grade Anti-EGFRvIII Antibody (AMG-595) |
HF004516 |
ELISA, FACS, Functional assay |
|
|
Anti-Human BRAF/B-Raf (V600E) Antibody (SAA2181) |
HB617033 |
ELISA |
|
|
Anti-BRAF Polyclonal Antibody |
HB617014 |
ELISA, IHC, WB |
|
|
Anti-VEGFC Polyclonal Antibody |
HW305014 |
ELISA, IHC, WB |
|
|
Research Grade Anti-PD-L1 & VEGF Bispecific Antibody (Pm8002) |
HB941236 |
ELISA, Bioactivity: FACS, Functional assay, Research in vivo |
|
|
InVivoMAb Anti-Mouse TIGIT & PD-L1 Bispecific Antibody |
MS739010 |
FuncS |
|
|
Anti-Human CD274/PD-L1/B7-H1 Antibody (2.7A4) |
HV974023 |
Blocking, ELISA, FCM |
|
|
Research Grade Anti-Human CD340/ERBB2/HER2/NEU (MEDI4276) |
HY286566 |
ELISA, Bioactivity: FACS, Functional assay, Research in vivo |
|
|
Anti-CD340/ERBB2/HER2/NEU Polyclonal Antibody |
HY286014 |
ELISA, IHC, WB |
|
|
Anti-Human CLDN18.2 Antibody (hu7V3) |
HX126013 |
ELISA |
abinScience提供的一系列结直肠癌研究相关的Recombinant Protein、Antibody及Research Biosimilar产品,涵盖了多个关键靶点,如需更多信息,欢迎扫码联系专属顾问!
[1] Al-Joufi FA, Setia A, Salem-Bekhit MM, Sahu RK, Alqahtani FY, Widyowati R, Aleanizy FS. Molecular Pathogenesis of Colorectal Cancer with an Emphasis on Recent Advances in Biomarkers, as Well as Nanotechnology-Based Diagnostic and Therapeutic Approaches. Nanomaterials (Basel). 2022 Jan 4;12(1):169.
[2] Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, Gandini S, Lizier M, Braga D, Asnicar F, Segata N, Klaver C, Brescia P, Rossi E, Anselmo A, Guglietta S, Maroli A, Spaggiari P, Tarazona N, Cervantes A, Marsoni S, Lazzari L, Jodice MG, Luise C, Erreni M, Pece S, Di Fiore PP, Viale G, Spinelli A, Pozzi C, Penna G, Rescigno M. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021 May 10;39(5):708-724.e11.
[3] Tilg H, Adolph TE, Gerner RR, Moschen AR. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018 Jun 11;33(6):954-964.
[4] Chen Y, Wang D, Li Y, Qi L, Si W, Bo Y, Chen X, Ye Z, Fan H, Liu B, Liu C, Zhang L, Zhang X, Li Z, Zhu L, Wu A, Zhang Z. Spatiotemporal single-cell analysis decodes cellular dynamics underlying different responses to immunotherapy in colorectal cancer. Cancer Cell. 2024 Jul 8;42(7):1268-1285.e7.
[5] Li J, Wu C, Hu H, Qin G, Wu X, Bai F, Zhang J, Cai Y, Huang Y, Wang C, Yang J, Luan Y, Jiang Z, Ling J, Wu Z, Chen Y, Xie Z, Deng Y. Remodeling of the immune and stromal cell compartment by PD-1 blockade in mismatch repair-deficient colorectal cancer. Cancer Cell. 2023 Jun 12;41(6):1152-1169.e7.
[6] Wang X, Fang Y, Liang W, Wong CC, Qin H, Gao Y, Liang M, Song L, Zhang Y, Fan M, Liu C, Lau HC, Xu L, Li X, Song W, Wang J, Wang N, Yang T, Mo M, Zhang X, Fang J, Liao B, Sung JJY, Yu J. Fusobacterium nucleatum facilitates anti-PD-1 therapy in microsatellite stable colorectal cancer. Cancer Cell. 2024 Oct 14;42(10):1729-1746.e8.
[7] Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023 May-Jun;73(3):233-254. doi: 10.3322/caac.21772. Epub 2023 Mar 1.
[8] Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016 Mar;16(3):173-86. doi: 10.1038/nrc.2016.4. Epub 2016 Feb 12.
[9] Ashktorab H, Brim H. Colorectal cancer subtyping. Nat Rev Cancer. 2022 Feb;22(2):68-69.
[10] Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017 Feb;17(2):79-92. doi: 10.1038/nrc.2016.126. Epub 2017 Jan 4. Erratum in: Nat Rev Cancer. 2017 Mar 23;17(4):268
返回顶部